In this article, we will explore which molecules show an appropriate number of bonds and why it is important for their stability. 1. Water (H2O): Water is a molecule composed of two hydrogen atoms and one oxygen atom. Oxygen has six valence electrons, and hydrogen has one. Oxygen forms two bonds with hydrogen atoms, resulting in a stable molecule.
Full article: Fluorophore tagged bio-molecules and their applications: A brief review
Dec 4, 2023question No one rated this answer yet — why not be the first? 😎 SwethaJ Final answer: Methane, ethene, ethyne, and benzene are hydrocarbons where each carbon atom has four covalent bonds, accordingly fulfilling carbon’s bonding requirements.

Source Image: shutterstock.com
Download Image
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a “skeleton structure.”.

Source Image: sciencenews.org
Download Image
Mastering Biology 1 – 5 Flashcards | Quizlet Which molecules show an appropriate number of bonds around each carbon atom? Click the card to flip 👆 Since carbon atoms are tetravalent (able to form four bonds), atoms may branch off a carbon atom in as many as four places. Click the card to flip 👆 1 / 56 Flashcards Learn Test Match Q-Chat Created by britneygiang Students also viewed

Source Image: novapublishers.com
Download Image
Which Molecules Show An Appropriate Number Of Bonds
Which molecules show an appropriate number of bonds around each carbon atom? Click the card to flip 👆 Since carbon atoms are tetravalent (able to form four bonds), atoms may branch off a carbon atom in as many as four places. Click the card to flip 👆 1 / 56 Flashcards Learn Test Match Q-Chat Created by britneygiang Students also viewed Sep 12, 2023In chemistry, carbon typically forms four bonds. Examples of molecules with accurate number of bonds around each carbon atom would be methane (CH4) and ethane (C2H6) where each carbon atom forms four bonds. Functional groups in organic chemistry are groups of atoms that have specific chemical properties.
Water in Biology: A Molecular View – Nova Science Publishers
Biology Biology questions and answers Which molecules show an appropriate number of bonds around each carbon atom? Select the three that apply. This problem has been solved! You’ll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer Explainer: In chemistry, what does it mean to be organic?

Source Image: snexplores.org
Download Image
Quinoa: The Protein Powerhouse for Natural and Healthy Hair – Camille Rose Naturals Biology Biology questions and answers Which molecules show an appropriate number of bonds around each carbon atom? Select the three that apply. This problem has been solved! You’ll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer

Source Image: camillerose.com
Download Image
Full article: Fluorophore tagged bio-molecules and their applications: A brief review In this article, we will explore which molecules show an appropriate number of bonds and why it is important for their stability. 1. Water (H2O): Water is a molecule composed of two hydrogen atoms and one oxygen atom. Oxygen has six valence electrons, and hydrogen has one. Oxygen forms two bonds with hydrogen atoms, resulting in a stable molecule.

Source Image: tandfonline.com
Download Image
Mastering Biology 1 – 5 Flashcards | Quizlet Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a “skeleton structure.”.

Source Image: quizlet.com
Download Image
Scrambling molecules with light – Combustion Research Facility Science Biology Biology questions and answers Which molecules show an appropriate number of bonds around each carbon atom? Select the three that apply. View Available Hint (s) This problem has been solved! You’ll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer

Source Image: crf.sandia.gov
Download Image
Identify the most polar bond in each molecule. (a) HSCH2CH2OH (b) CHCl2F (c) HOCH2CH2NH2 | Homework.Study.com Which molecules show an appropriate number of bonds around each carbon atom? Click the card to flip 👆 Since carbon atoms are tetravalent (able to form four bonds), atoms may branch off a carbon atom in as many as four places. Click the card to flip 👆 1 / 56 Flashcards Learn Test Match Q-Chat Created by britneygiang Students also viewed

Source Image: homework.study.com
Download Image
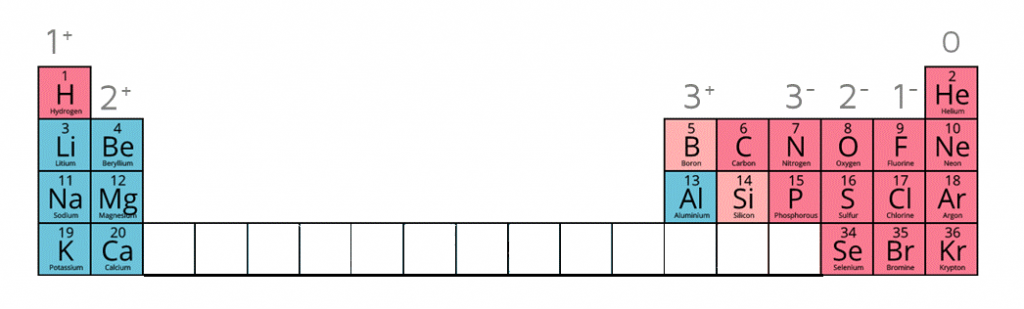
1:38 know the charges of these ions: metals in Groups 1, 2 and 3, non-metals in Groups 5, 6 and 7, Ag⁺, Cu²⁺, Fe²⁺, Fe³⁺, Pb²⁺, Zn²⁺, hydrogen (H⁺), hydroxide (OH⁻), ammonium ( Sep 12, 2023In chemistry, carbon typically forms four bonds. Examples of molecules with accurate number of bonds around each carbon atom would be methane (CH4) and ethane (C2H6) where each carbon atom forms four bonds. Functional groups in organic chemistry are groups of atoms that have specific chemical properties.
Source Image: tutormyself.com
Download Image
Quinoa: The Protein Powerhouse for Natural and Healthy Hair – Camille Rose Naturals
1:38 know the charges of these ions: metals in Groups 1, 2 and 3, non-metals in Groups 5, 6 and 7, Ag⁺, Cu²⁺, Fe²⁺, Fe³⁺, Pb²⁺, Zn²⁺, hydrogen (H⁺), hydroxide (OH⁻), ammonium ( Dec 4, 2023question No one rated this answer yet — why not be the first? 😎 SwethaJ Final answer: Methane, ethene, ethyne, and benzene are hydrocarbons where each carbon atom has four covalent bonds, accordingly fulfilling carbon’s bonding requirements.
Mastering Biology 1 – 5 Flashcards | Quizlet Identify the most polar bond in each molecule. (a) HSCH2CH2OH (b) CHCl2F (c) HOCH2CH2NH2 | Homework.Study.com Science Biology Biology questions and answers Which molecules show an appropriate number of bonds around each carbon atom? Select the three that apply. View Available Hint (s) This problem has been solved! You’ll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer