Lesson 1: Diels–Alder reaction Diels–Alder reaction Diels–Alder: stereochemistry of dienophile Diels–Alder: stereochemistry of diene Diels–Alder: endo rule Diels–Alder: intramolecular Diels–Alder: regiochemistry Science > Organic chemistry > Conjugated systems and pericyclic reactions > Diels–Alder reaction
Solved Predict the major product of the following Diels- | Chegg.com
Predict the products of the following Diels–Alder reactions. (a)… | Channels for Pearson+ Organic Chemistry 16. Conjugated Systems Diels–Alder Reaction Problem 15d Textbook Question Predict the products of the following Diels–Alder reactions. (a) (b) Verified Solution
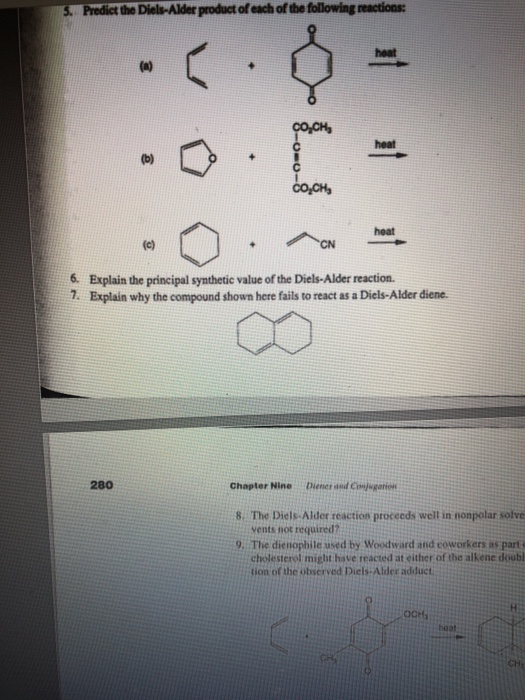
Source Image: chegg.com
Download Image
The Diels–Alder reaction is most useful for synthesizing molecules in the lab. But scientists believe that specific enzymes catalyze Diels–Alder reactions in some organisms. Examples are the formation of lovastatin, a cholesterol-lowering drug found in oyster mushrooms, and Spinosyn A, a natural insecticide produced by a certain bacterium. Comment.

Source Image: pearson.com
Download Image
Solved 1. Predict the product of the following Diels-Alder | Chegg.com Predict the Products of the Diels–Alder Reaction with Practice Problems – Chemistry Steps Organic Chemistry The Diels–Alder Reaction Predict the Products of the Diels–Alder Reaction with Practice Problems Practice 1. Predict the major product (s) with correct stereochemistry for each of the following Diels–Alder reactions: a) answer b) answer

Source Image: pearson.com
Download Image
Predict The Product For The Following Diels-Alder Reaction
Predict the Products of the Diels–Alder Reaction with Practice Problems – Chemistry Steps Organic Chemistry The Diels–Alder Reaction Predict the Products of the Diels–Alder Reaction with Practice Problems Practice 1. Predict the major product (s) with correct stereochemistry for each of the following Diels–Alder reactions: a) answer b) answer The Diels–Alder cycloaddition reaction occurs most rapidly if the alkene component, called the dienophile (“diene lover”), has an electron-withdrawing substituent group. Thus, ethylene itself reacts sluggishly, but propenal, ethyl propenoate, maleic anhydride, benzoquinone, propenenitrile, and similar compounds are highly reactive.
Predict the products of the following proposed Diels–Alder reacti… | Channels for Pearson+
Sep 29, 2023Predict the product of the following Diels–Alder reaction: Strategy Draw the diene so that the ends of its two double bonds are near the dienophile double bond. Then form two single bonds between the partners, convert the three double bonds into single bonds, and convert the former single bond of the diene into a double bond. Solved QUESTION 17 Predict the product of Diels-Alder | Chegg.com
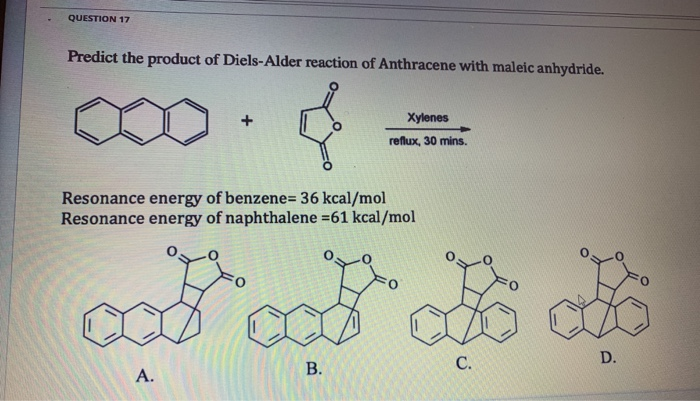
Source Image: chegg.com
Download Image
The Diels-Alder Reaction | MendelSet Sep 29, 2023Predict the product of the following Diels–Alder reaction: Strategy Draw the diene so that the ends of its two double bonds are near the dienophile double bond. Then form two single bonds between the partners, convert the three double bonds into single bonds, and convert the former single bond of the diene into a double bond.
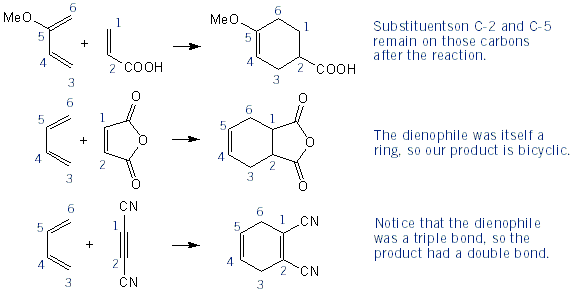
Source Image: mendelset.com
Download Image
Solved Predict the major product of the following Diels- | Chegg.com Lesson 1: Diels–Alder reaction Diels–Alder reaction Diels–Alder: stereochemistry of dienophile Diels–Alder: stereochemistry of diene Diels–Alder: endo rule Diels–Alder: intramolecular Diels–Alder: regiochemistry Science > Organic chemistry > Conjugated systems and pericyclic reactions > Diels–Alder reaction
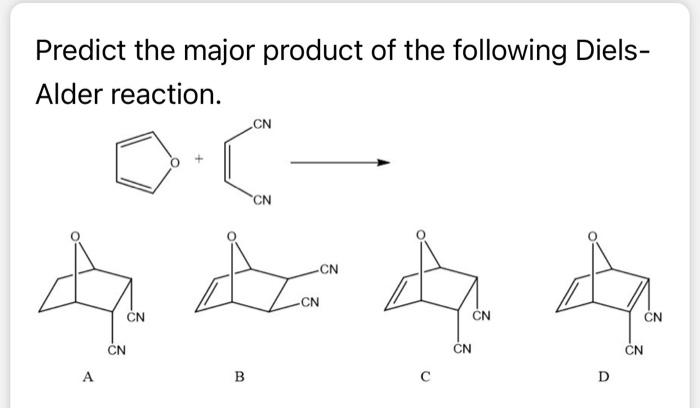
Source Image: chegg.com
Download Image
Solved 1. Predict the product of the following Diels-Alder | Chegg.com The Diels–Alder reaction is most useful for synthesizing molecules in the lab. But scientists believe that specific enzymes catalyze Diels–Alder reactions in some organisms. Examples are the formation of lovastatin, a cholesterol-lowering drug found in oyster mushrooms, and Spinosyn A, a natural insecticide produced by a certain bacterium. Comment.

Source Image: chegg.com
Download Image
Solved Predict the product for the following Diels-Alder | Chegg.com 1. Predict the product of the following Diels–Alder reactions; under kinetic control. Include the stereochemistry where appropriate. 2. Furan and maleimide, shown below, react to produce and adduct via a Diels–Alder reaction. At 25°C the isomer produced is the endo product, however at 90°C the exo isomer predominates.
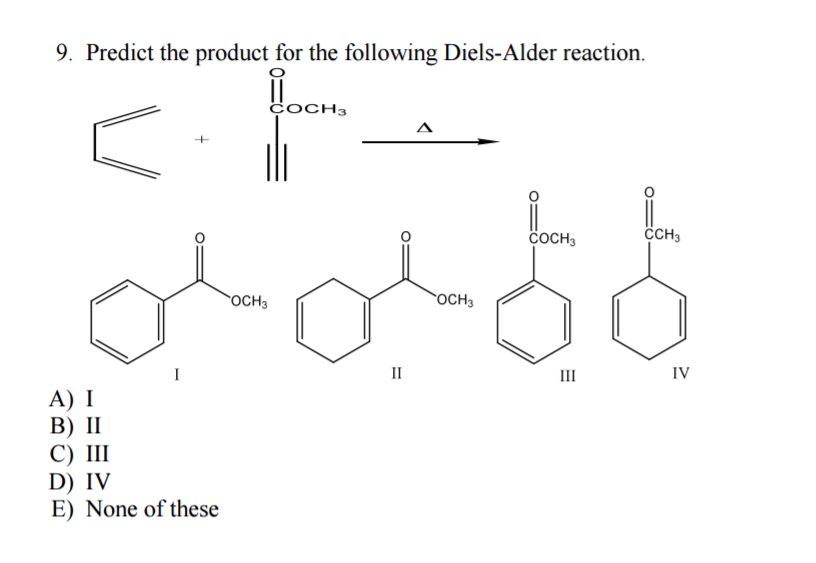
Source Image: chegg.com
Download Image
What dienophiles would react to give the following Diels-Alder products? | Homework.Study.com Predict the Products of the Diels–Alder Reaction with Practice Problems – Chemistry Steps Organic Chemistry The Diels–Alder Reaction Predict the Products of the Diels–Alder Reaction with Practice Problems Practice 1. Predict the major product (s) with correct stereochemistry for each of the following Diels–Alder reactions: a) answer b) answer

Source Image: homework.study.com
Download Image
Solved] . 9. Predict the major product for the following Diels-Alder… | Course Hero The Diels–Alder cycloaddition reaction occurs most rapidly if the alkene component, called the dienophile (“diene lover”), has an electron-withdrawing substituent group. Thus, ethylene itself reacts sluggishly, but propenal, ethyl propenoate, maleic anhydride, benzoquinone, propenenitrile, and similar compounds are highly reactive.
Source Image: coursehero.com
Download Image
The Diels-Alder Reaction | MendelSet
Solved] . 9. Predict the major product for the following Diels-Alder… | Course Hero Predict the products of the following Diels–Alder reactions. (a)… | Channels for Pearson+ Organic Chemistry 16. Conjugated Systems Diels–Alder Reaction Problem 15d Textbook Question Predict the products of the following Diels–Alder reactions. (a) (b) Verified Solution
Solved 1. Predict the product of the following Diels-Alder | Chegg.com What dienophiles would react to give the following Diels-Alder products? | Homework.Study.com 1. Predict the product of the following Diels–Alder reactions; under kinetic control. Include the stereochemistry where appropriate. 2. Furan and maleimide, shown below, react to produce and adduct via a Diels–Alder reaction. At 25°C the isomer produced is the endo product, however at 90°C the exo isomer predominates.